Please sign in first
Not a member?

- Home
- Online Education Programs
- ISG Journey Thru Gemology
- ISG Credentials
- Legacy Entrance
- Frequently Asked Questions
- Registry of Graduates
- Careers in Gemology
- Important Program Information
- About the ISG
- Why is the ISG Tuition So Low?
- Testimonials of ISG Students and Graduates
- Meet Your Instructor for your ISG education
- ISG Best Online Gemology School
- Graduate Support Programs
- Ishihara Color Vision Test
- Privacy Policy
- Newsletters
- The Story of Ruby
- Turquoise Investigation by the ISG
- The Story of Petrol Quartz
- Top 5 Myths About Jewelry Insurance Appraisals
- The Story of Time
- The Story of Freshwater Pearls
- The Story of Agates and Jaspers
- Susan Bailey v. Frantz Jewelers, et al.
- The Story of Blue Topaz
- The Story of Tibet Andesine
- The Story of Oregon Sunstone
- The Story of Created Moissanite
- Identifying Lab Created Diamonds
- Black Diamond or Created Moissanite?
- Ebay, the GIA, and Section 230
- Lessons From the Angry Janitor
- Exposing the Truth about Lab-Created Diamonds
- AGTA Hobbles Dealers and Buyers
- Appraisers -v- Gem Labs…a Legal Imbalance!
- Understanding the Classification of Diamonds
- Cremation Diamonds: Insuring and Litigating
- Let’s Talk About Gemology Credentials
- Hometown Jewelers and the Force Majeure Clause
- Following the Critical Angle of Diamond Grading
- When Breitling Brought the Airmen Home
- Let’s Open a Refractometer
- Measuring the Energy of Light
- Quick Reference Guide to Sunstone
- ISG Seeing the Invisible Light
- Contact Us
Measuring the Energy of Light
Measuring the Energy of Light
A couple of weeks ago we looked at the electromagnetic spectrum of visible light and using our MDM UV-VIS-NIR Spectrometer were able to see the invisible light. The spectrum beyond what our eyes can normally see. Today, I would like to provide a simple demonstration of the variable energies of light that allow gemstones to selectively absorb certain energy levels to create the amazing colors we see. While we cannot actually measure the energy levels with our limited resources, by getting a bit creative we can, in fact, demonstrate that light has variable energies. In our case, thermal energy.
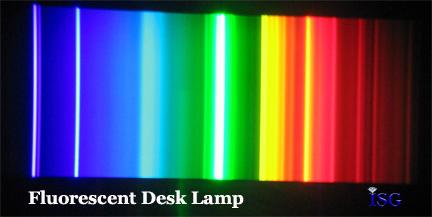 A full spectrum light gives a gemstone a complete set of energy levels to use in order to produce one or more colors that we see. Use a light with a limited spectrum, such as a fluorescent desk light, and you don’t give the gemstone a full set of “tools” to work with to produce colors. As we have seen with the spectra of difference gemstones, each produces its own specific color based on selectively absorbing certain colors while passing the rest through to our eyes where we perceive the colors.
A full spectrum light gives a gemstone a complete set of energy levels to use in order to produce one or more colors that we see. Use a light with a limited spectrum, such as a fluorescent desk light, and you don’t give the gemstone a full set of “tools” to work with to produce colors. As we have seen with the spectra of difference gemstones, each produces its own specific color based on selectively absorbing certain colors while passing the rest through to our eyes where we perceive the colors.
We can carry this demonstration one step further and actually measure one type of energy of light using a simple prism, a baby thermometer, and an hour in the back yard of our ISG office. While the physics of this is far above my pay grade, as usual I try to find simple ways to demonstrate otherwise technical issues so that those of us without PhD’s in physics can understand the concept.
The Concept: Since each color of the spectrum represents a slightly different energy level, each color should produce a slightly different energy reaction when tested. In our case, we are going to test thermal energy. This will only deal with the heat produced by the spectrum of colors and nothing else. So please don’t any physics PhD guys contact me about some high-tech physics of electron volts and all of that. I realize it takes, for instance, 2.5eV for chromium to produce a red color in ruby (thank you Gem-A) but for our purposes we are going to keep it simple and measure the heat differences of spectrum colors.
 The Set Up: In order to compare the variable thermal energy, or heat, of the spectrum, we must first create a spectrum of light strong enough to be measurable thermal energy.
The Set Up: In order to compare the variable thermal energy, or heat, of the spectrum, we must first create a spectrum of light strong enough to be measurable thermal energy.
For this purpose I used a chair, a prism, a baby thermometer and paper. Since the strongest spectrum I could create is the spectrum of the sun, I went outside and worked for a half hour to orient this set up just right to produce the spectrum of the sun on a paper, using the small prism as you see at left on the top of the back of this chair.
But there were problems that had to be resolved before this would work…

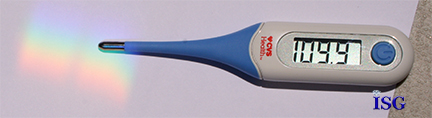 While the spectrum is fairly easy to create, the problem is measuring the thermal temperature in direct sunlight negates the ability to measure the temperature of the individual color. The ambient temperature in the shade on this day was a confirmed 87 degrees F. But in direct sunlight, the thermometer measured 109.9F as seen below. So, the trick was to have the prism in the direct sunlight to produce a complete and full spectrum, and yet have the spectrum projected in the shade where the ambient temperature would be equal and allow measurement of each color of the spectrum.
While the spectrum is fairly easy to create, the problem is measuring the thermal temperature in direct sunlight negates the ability to measure the temperature of the individual color. The ambient temperature in the shade on this day was a confirmed 87 degrees F. But in direct sunlight, the thermometer measured 109.9F as seen below. So, the trick was to have the prism in the direct sunlight to produce a complete and full spectrum, and yet have the spectrum projected in the shade where the ambient temperature would be equal and allow measurement of each color of the spectrum.
 This required a bit of moving and testing to get the seat of the chair under the shade of a Texas Live Oak tree, while keeping the back of the chair with the prism in the direct sunlight. By carefully placing the set up as you see at left, I was able to measure the temperature or thermal energy of each end of the visible spectrum in the controlled ambient temperature of the shade.
This required a bit of moving and testing to get the seat of the chair under the shade of a Texas Live Oak tree, while keeping the back of the chair with the prism in the direct sunlight. By carefully placing the set up as you see at left, I was able to measure the temperature or thermal energy of each end of the visible spectrum in the controlled ambient temperature of the shade.
Here are the results that demonstrate how different colors in the visible light spectrum will offer variable temperatures, and how that allows us to measure at least one very basic energy level of light.
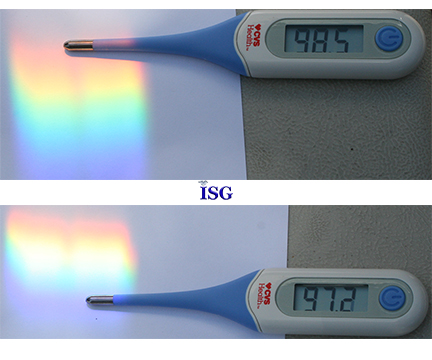 Here you see the temperature readings from the far-red side of the spectrum to the indigo or blue side. We know the ambient temperature in the shade at this time was 87 degrees F. The blue end measures 97.2 F while the red end measures 98.5F, or a difference of 1.3 degrees F in this spectrum of colors from the sun, taken at the same place and same time of day, just moving the thermometer from one side to the other.
Here you see the temperature readings from the far-red side of the spectrum to the indigo or blue side. We know the ambient temperature in the shade at this time was 87 degrees F. The blue end measures 97.2 F while the red end measures 98.5F, or a difference of 1.3 degrees F in this spectrum of colors from the sun, taken at the same place and same time of day, just moving the thermometer from one side to the other.
We know the direct sunlight was 109.9 F, and the ambient temperature in the shade was 87F, so the difference in this thermometer reading between these two colors demonstrates the difference in thermal or heat energy of these two color regions within this spectrum.
OK, one more.
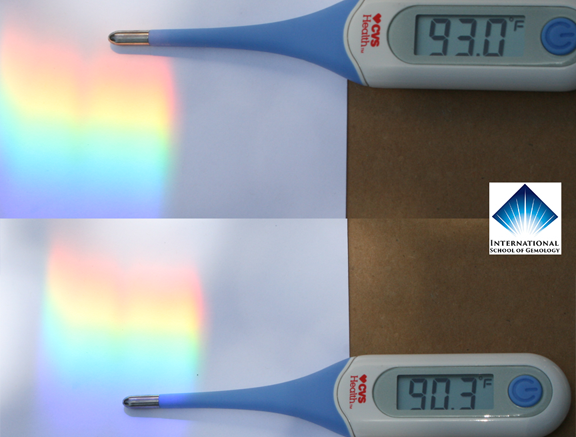
Just to make sure this demonstration was predictable and repeatable, I waited until later in the day and performed the same measurement. The results are below. The ambient temperature in the shade had fallen to around 80 degrees F. Below are the new readings in the later afternoon sunlight.
 As you can see, 91.2F in the blue end, and 92.9F in the red. A difference of 1.7 degrees F between these two sides of the spectrum.
As you can see, 91.2F in the blue end, and 92.9F in the red. A difference of 1.7 degrees F between these two sides of the spectrum.
The concept that the red colors generate more heat is no magical, mystery information. Anyone who has lived or traveled to the Caribbean and seen so many of the houses with red tile roofs understands that reflecting the red color of the spectrum is also reflecting heat off the roof of the house. But that is a side issue to the whole thing, but one that I thought I would throw in.
Below is another combination image of another confirmation test I ran later. Pretty amazing to see the difference in the thermal energy.
Bottom line is that elements inside a gemstone utilize the variable energy levels of light to produce the amazing colors we see. Chromium uses light to produce red in rubies, green in emeralds, and red and green in alexandrites. The list could go on and on. Gemstones need a complete spectrum of light to produce the most amazing colors. In this demonstration we have seen just a tiny part of this amazing story. By measuring the thermal energy of light in this small way, we can better understand how light and gemstones interact to produce beautiful colors in nature’s most wonderous gifts….colored gemstones.
